State of the art
Since 2015, endovascular thrombectomy (EVT), with or without prior intravenous thrombolysis, is the standard of care of acute ischemic stroke (AIS) treatment due to an anterior circulation large vessel occlusion (LVO) in selected patients with small ischemic core (ASPECTS 6-10).1, 2 The number needed to treat for one patient to have reduced disability of at least 1 point on mRS was 2.6.2 Two years later, clinical benefit of EVT within 24 hours was also demonstrated with the positive results of the DAWN and DEFUSE 3 trials.3, 4 The number needed to treat for one patient to have a reduced disability score by one or more values on the mRS was 3. And in 2023, several randomized controlled trials showed the clinical benefit of EVT in patients with large ischemic core, mostly defined by ASPECTS 3-5.5, 6, 7The LASTE trial demonstrated also the benefit of EVT in very large core, defined as ASPECTS 0-5.8
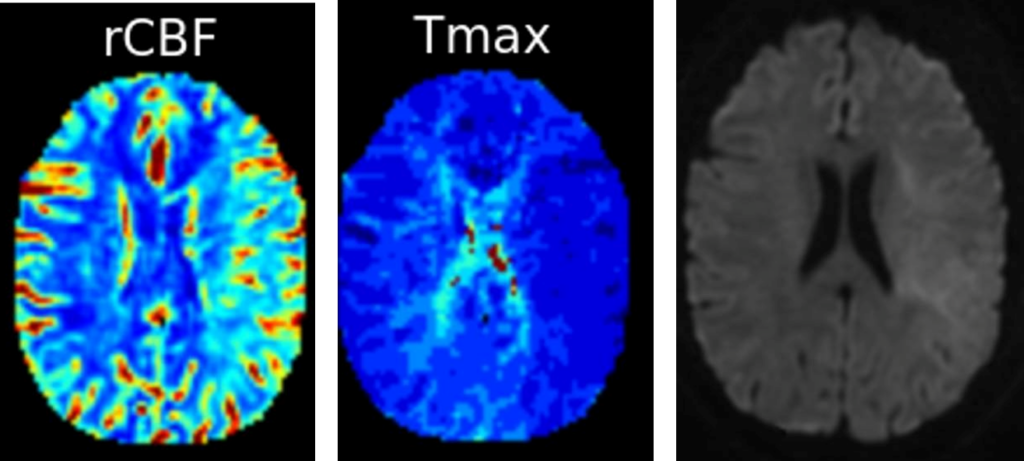
Whereas there is a strong association between achieved reperfusion grade and functional outcome, successful reperfusion (eTICI 2b-3) after EVT was achieved in 70% in the pooled patient-level data of the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration and a complete reperfusion (eTICI 3) occurs in only 30%.2 In addition, up to 40% of patients presented no-reflow (NR) phenomenon, a failure of downstream microvascular reperfusion, after successful EVT.9, 10 Adjunct intra-arterial (IA) thrombolysis may be a promising therapeutic option allowing recanalization of distal arterial occlusions, not accessible to mechanical devices, and improvement of upstream brain reperfusion by targeting microvascular obstruction.
Alteplase and IA thrombolysis trials
Existing literature regarding IA thrombolysis in the new era of EVT is limited to observational and/or retrospective studies with small sample size and heterogenous dosing regimen. A meta-analysis found that EVT with IA alteplase did not improve the recanalization rate but provided better functional outcomes.11 One randomized trial, phase 2b CHOICE trial, was performed and published in 2022.12 Among 113 included patients, 61 received IA injection of Alteplase (0.225mg/kg) at the end of EVT with final eTICI 2b-3. They found that patients receiving IA Alteplase presented a better clinical outcome with no safety concerns. Adjunct alteplase enhances brain reperfusion, which results in reduced expansion of the infarction and improved neuronal integrity.13 Severals randomized trials testing the clinical and safety outcomes of IA thrombolysis (Alteplase or Tenecteplase) post successful EVT are ongoing or will start soon. IA-SUCCESS and CHOICE2 (NCT05797792) are the 2 trials testing the IA Alteplase. CHOICE2 is a multicenter, randomized, parallel-group, superiority study including 440 patients. The primary outcome is the proportion of patients with microvascular hypoperfusion on CT Perfusion at 36±24h. The key secondary outcome is the proportion of patients with a mRS 0 to 1 at 90 days. TECNO (NCT05499832), ANGEL-TNK (NCT05624190), EXTEND-AGNES TNK (NCT05892510) and BRETIS-TNK II (NCT05657444) are testing IA Tenecteplase.
Rational
Despite successful angiographic reperfusion, still 50% of acute ischemic stroke patients with anterior circulation large vessel occlusion are functionally independent at 90 days. IA-SUCCESS trial aims to determine whether the intra-arterial thrombolysis is efficient as an add-on to endovascular thrombectomy ± prior intravenous thrombolysis in the setting of successful brain reperfusion on cerebral angiogram, allowing recanalization of distal arterial occlusions and improvement of upstream brain reperfusion by targeting microvascular obstruction.
Objectives
The primary outcome is the severity of disability according to the distribution of the modified Rankin Scale (mRS) score at 90 ± 15 days, assessed by an independent qualified nurse certified in the assessment of the mRS and blinded to the randomization group. The key secondary outcomes are excellent (mRS 0-1) and favorable outcome (mRS 0-2) at 90 days, symptomatic intracranial hemorrhage and 90-day mortality.
Design and outcomes
IA-SUCCESS is a prospective phase 3 multicenter open label blinded endpoint (PROBE) superiority randomized controlled trial, with health-economics evaluation. The objective is to demonstrate the superiority of adjunct IA thrombolysis compared to no adjunct IA thrombolysis after successful angiographic reperfusion (eTICI 2b-3) after IVT alone, bridging therapy, or EVT alone.
The sample size calculation is based on the common odds ratio for better functional outcome (90-day mRS score). 626 patients should be included.
The primary endpoint is the severity of disability according to the distribution of scores on the modified Rankin Scale (mRS) at 90 (±15) days, assessed by central blinded and qualified nurse certified in the assessment of the mRS following a structured questionnaire of the mRS. The secondary key outcomes are mRS 0-1, mRS 0-2 and mortality at 90 days, any intracerebral hemorrhage, symptomatic intracerebral hemorrhage, infarct growth and procedural complications.
Key inclusion criteria
- Age ≥ 18 years
- Pre-stroke mRS 0-2
- Acute ischemic stroke with anterior circulation LVO defined as intracranial internal carotid artery, M1, or M2 occlusion proven on CT or MRI
- NIHSS score ≥ 5 at admission
- Acute reperfusion strategy started within 24h after stroke onset according to the international guidelines
- DWI-ASPECTS ≥ 2 (MRI) or CT-ASPECTS ≥ 3
- Delay from imaging to puncture within 3 hours for transferred patients
- eTICI 2b-2c-3 after IVT alone, bridging therapy (IVT + MT), or MT alone and confirmed by catheter angiogram
- Person affiliated to or beneficiary of a social security plan
Key exclusion criteria
- Person who do not speak French
- Contraindications for IA thrombolysis: Platelet count <100 000/mm3, INR >1.7, AOD use <48h or biological confirmation of activity and effective heparin treatment
- Bleeding-risk complications during the mechanical thrombectomy procedure (e.g carotid dissection, complicated femoral approach)
- Bleeding-risk complications consecutive to a fall associated with stroke
- More than 5 thrombectomy device
- Intracerebral hemorrhage
- Occlusion or high grade stenosis treated by stenting
- Patient expected to be unable to present or be available for 3-month visit follow-up
- Participation in another clinical trial within 30 days prior to the inclusion which the experiment may affect the 90-day mRS score
- Woman of childbearing age without effective contraception
- Person referred in articles L.1121-5, L. 1121-7 and L.1121-8 of the French Public Health Code
Data Safety Monitoring Board

Laurent DEREX
MD, PhDDepartment of Neurology
Hospices Civils de Lyon

Sébastien SOIZE
MD, PhDDepartment of Neuroradiology
University Hospital of Reims

Julien LABREUCHE
PhDDepartment of Biostatistics
University Hospital of Lille
Steering Committee

Benjamin GORY
Professor
Sébastien RICHARD
Professor
Guillaume TURC
Professor
Gabriela HOSSU
Phd
Marie-Lauren ANTOINE
Doctor
Jean-Marc OLIVOT
Professor
Mikael MAZIGHI
Professor
Gaultier MARNAT
Professor
Nicolas BRICOUT
Doctor
Igor SIBON
Professor
Marie TOUSSAINT-HACQUARD
Doctor
Maxime GAUBERTI
Doctor© Alexandre Darmon / Art in Research pour la Fondation Bettencourt Schueller

Christophe COGNARD
ProfessorReferences
- Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. ↩︎
- Goyal M, Menon BK, Van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. ↩︎
- Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21. ↩︎
- Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. ↩︎
- Yoshimura S, Sakai N, Yamagami H, et al. Endovascular Therapy for Acute Stroke with a Large Ischemic Region.N Engl J Med. 2022;386:1303-1313. ↩︎
- Sarraj A, Hassan AE, Abraham MG, et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N Engl J Med. 2023;388:1259-1271. ↩︎
- Bendszus M, Fiehler J, Subtil F, et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: multicentre, open-label, randomised trial. Lancet. 2023;402:1753-1763. ↩︎
- Costalat V, Lapergue B, Albucher JF, et al. Evaluation of acute mechanical revascularization in large stroke (ASPECTS ⩽5) and large vessel occlusion within 7 h of last-seen-well: The LASTE multicenter, randomized, clinical trial protocol. Int J Stroke. 2024;19:114-119. ↩︎
- Ng FC, Churilov L, Yassi N, et al. Prevalence and significance of impaired microvascular tissue reperfusion despite macrovascular angiographic reperfusion (no-reflow). Neurology. 2022;98:e790-e801. ↩︎
- Schiphorst AT, Turc G, Hassen WB, et al. Incidence, severity and impact on functional outcome of persistent hypoperfusion despite large-vessel recanalization, a potential marker of impaired microvascular reperfusion: Systematic review of the clinical literature. J Cereb Blood Flow Metab. 2023 Oct 23. Online ahead of print. ↩︎
- Yang X, Wang Z, Chen H, et al. Mechanical thrombectomy with intra-arterial alteplase provided better functional outcomes for AIS-LVO: a meta-analysis. Front Neurosci. 2023 Jul 6;17:1137543. ↩︎
- Renu A, Millan M, San Roman L, et al. Effect of intra-arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: The choice randomized clinical trial. JAMA; 2022;327:826-835. ↩︎
- Laredo C, Rodríguez A, Oleaga L, et al. Adjunct Thrombolysis Enhances Brain Reperfusion following Successful Thrombectomy. Ann Neurol. 2022.92:860-870. ↩︎
